Despite the constant improvement in operation techniques, intensive care and standardized antibiotic use, postoperative complications are still a significant problem in daily clinical practice. Knowing which patients are at the most risk of developing these is vital for achieving the best possible outcomes.
In recent years, the use of next-generation sequencing (NGS) has changed our perception and understanding of the structure and function of the microbiome in various organ systems. The use of NGS allows new insights into the complex composition of the intestinal flora and its reaction to external influences.
We wanted to figure out if the gut microbiome experiences major changes in the perioperative setting, and if changes in bacterial diversity or composition might have an impact on the incidence of postoperative complications.
We analyzed 116 stool samples of 32 patients undergoing pancreatic surgery using 16S-rRNA gene next-generation sequencing. Based on the structure of the gut microbiome, the samples could be allocated into three different microbial communities (A, B and C).
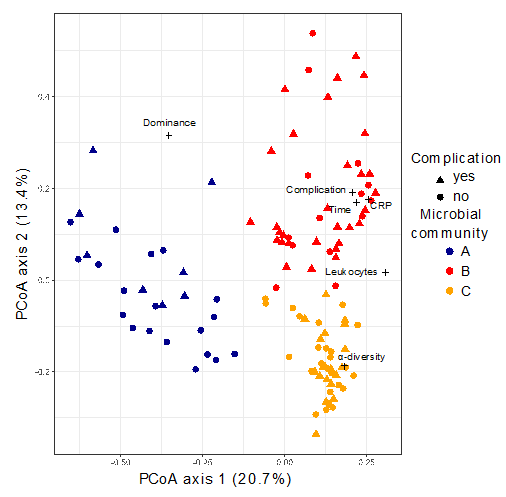
The composition of community B was characterised, among others, by an increase in Akkermansia that belongs to the Verrucomicrobia phylum which degrade intestinal gut mucin as their sole carbon and nitrogen source. The mucin layer of the intestinal gut has an important function as it acts as a physical barrier to protect epithelial cells from pathogen invasion.
Animal experiments have shown a rise in mucin production, along with an increase in Akkermansia in the gut microbiome under pro-inflammatory conditions and a protective effect on the epithelium.
In contrast Lachnospiraecae were decreased, normally associated with resistance against colonisation by more pathogenic bacteria including Clostridium difficile, which might indicate an increased susceptibility of the intestinal flora.
Interestingly patients showing a microbial composition resembling community B at least once during the observation period were found to have a significantly higher risk for developing postoperative complications (e.g., anastomotic insufficiency or pancreatic fistula). Patients with a complicated course showed significantly increased C-reactive protein (CRP) levels, a higher leucocyte count, prolonged hospital stay and longer time in the intensive care unit (ICU). Six patients were treated with percutaneous drainage and two patients underwent re-operation.
Our results showed that differences in the gut microbiome are associated with the development of postoperative complications. Thus, methods that take into account differences in the NGS-defined microbial communities might represent a useful diagnostic tool in future clinical practice.
NGS could improve our daily diagnostic procedures, especially in comparison to the mostly imprecise microbiologic culture based methods. But this requires clinical trials with large numbers of different patients with multiple diseases to evaluate which changes in the microbiome really need a therapeutic intervention and which are just a laboratory phenomenon.
Comments